Page 183 - AI for Good Innovate for Impact
P. 183
AI for Good Innovate for Impact
(continued)
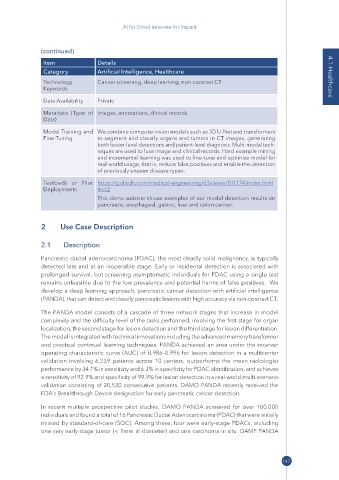
Item Details
Category Artificial Intelligence, Healthcare
Technology Cancer screening, deep learning, non-contrast CT 4.1-Healthcare
Keywords
Data Availability Private
Metadata (Type of Images, annotations, clinical records
Data)
Model Training and We combine computer vision models such as 3D U-Net and transformers
Fine-Tuning to segment and classify organs and tumors in CT images, generating
both lesion-level detections and patient-level diagnosis. Multi-modal tech-
niques are used to fuse image and clinical records. Hard example mining
and incremental learning was used to fine-tune and optimize model for
real-world usage, that is, reduce false positives and enable the detection
of previously unseen disease types.
i
Testbeds or Pilot https:// g .alicdn .com/ medical -engineering/ d3viewer/ 0 .0 .174/ ndex .html
Deployments #/ ct2
This demo website shows examples of our model detection results on
pancreatic, esophageal, gastric, liver and colon cancer.
2 Use Case Description
2�1 Description
Pancreatic ductal adenocarcinoma (PDAC), the most deadly solid malignancy, is typically
detected late and at an inoperable stage. Early or incidental detection is associated with
prolonged survival, but screening asymptomatic individuals for PDAC using a single test
remains unfeasible due to the low prevalence and potential harms of false positives. We
develop a deep learning approach, pancreatic cancer detection with artificial intelligence
(PANDA), that can detect and classify pancreatic lesions with high accuracy via non-contrast CT.
The PANDA model consists of a cascade of three network stages that increase in model
complexity and the difficulty level of the tasks performed, involving the first stage for organ
localization, the second stage for lesion detection and the third stage for lesion differentiation.
The model is integrated with technical innovations including the advanced memory transformer
and practical continual learning techniques. PANDA achieved an area under the receiver
operating characteristic curve (AUC) of 0.986–0.996 for lesion detection in a multicenter
validation involving 6,239 patients across 10 centers, outperforms the mean radiologist
performance by 34.1% in sensitivity and 6.3% in specificity for PDAC identification, and achieves
a sensitivity of 92.9% and specificity of 99.9% for lesion detection in a real-world multi-scenario
validation consisting of 20,530 consecutive patients. DAMO PANDA recently received the
FDA’s Breakthrough Device designation for early pancreatic cancer detection.
In recent multiple prospective pilot studies, DAMO PANDA screened for over 100,000
individuals and found a total of 16 Pancreatic Ductal Adenocarcinoma (PDAC) that were initially
missed by standard-of-care (SOC). Among these, four were early-stage PDACs, including
one very early-stage tumor (< 1mm in diameter) and one carcinoma in situ. DAMP PANDA
147